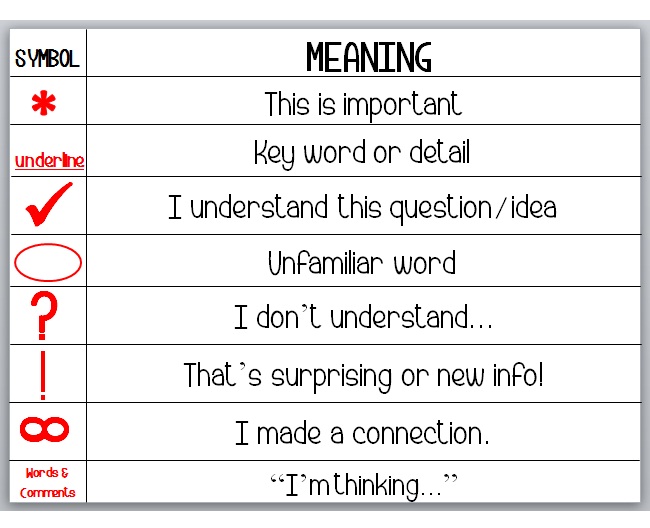
Here is some study material.
https://www.quia.com/quiz/303980.html
https://www.softschools.com/quizzes/science/physical_chemical_changes/quiz382.html
https://www.edinformatics.com/math_science/a_p_chem.htm
https://interactivesites.weebly.com/matter-chemical–physical.html